All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The Multiple Myeloma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Multiple Myeloma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Multiple Myeloma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Multiple Myeloma Hub cannot guarantee the accuracy of translated content. The Multiple Myeloma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Symposium | Looking to the future of bispecific antibodies in MM, and panelist Q&A
Bookmark this article
Video series
Multiple Myeloma Hub Virtual Symposium 2024: Current and future perspectives for bispecific antibodies
The Multiple Myeloma Hub virtual symposium held on March 11, 2024,“Current and future perspectives for bispecific antibodies in multiple myeloma: Learnings from 2023,” concluded by looking to the future of T-cell engagers in multiple myeloma, delivered by chair Sagar Lonial, Winship Cancer Institute of Emory University, Atlanta, US.
Lonial shared his future perspectives for the treatment of relapsed/refractory multiple myeloma, including strategies for managing resistance to B-cell maturation antigen, alternative treatments for anti-BCMA exposed patients, and the potential of cereblon E3 ligase modulators (Figure 1).
Figure 1. Novel CELMoD agents in development*
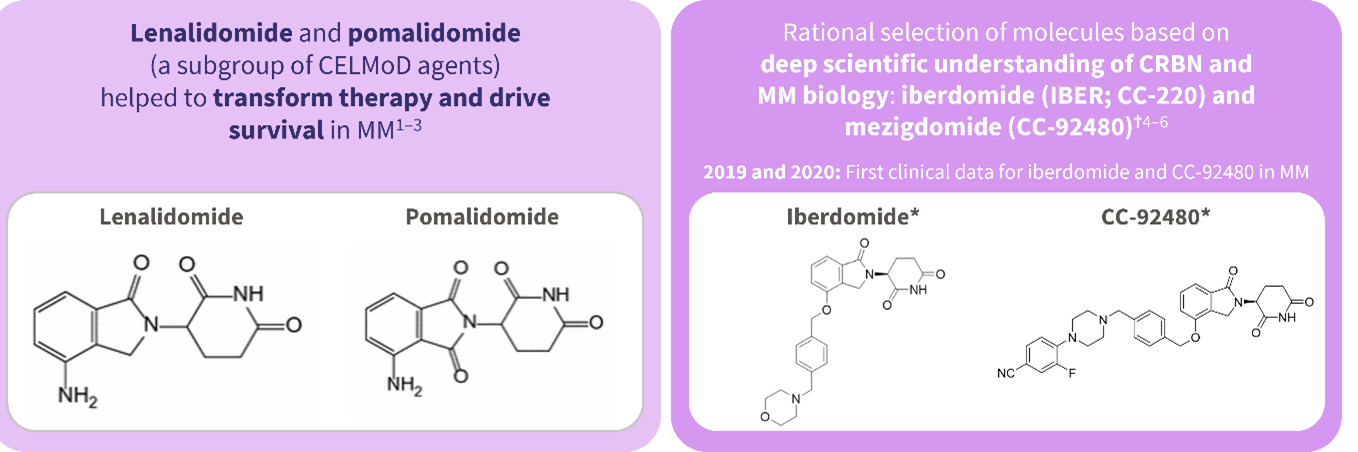
CELMoD, cereblon E3 ligase modulators; CRBN, cereblon; MM, multiple myeloma.
*Data from Rajkumar, et al.1; Facon, et al.2; Durie, et al.3; Ito and Handa.4; Matyskiela, et al.5; Hansen, et al.6
†Iberdomide (IBER; CC-220) and mezigdomide (CC-92480) are investigational products that are currently not approved by any regulatory agency.
Q&A session
This symposium closed with a panel Q&A session with live audience participation. Our panelists shared their perspectives on key topics including health-related quality of life in patients treated with bispecific antibodies, highlighting the impact of reduced dosing frequencies, as well as subcutaneous versus intravenous administration, on the patient experience.
This independent medical activity was funded by Janssen and Bristol Myers Squibb. All content was developed independently by the faculty. The funders were allowed no influence on the content of this activity.
Q&A session
- Rajkumar S, Jacobus S, Callander N, et al. Lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone as initial therapy for newly diagnosed multiple myeloma: an open-label randomised controlled trial. The Lancet. 2010;11(1):29-37. DOI: 1016/S1470-2045(09)70284-0
- Facon T, Dimopoulos M, Dispenzieri A, et al. Final analysis of survival outcomes in the phase 3 FIRST trial of up-front treatment for multiple myeloma. 2018;131(3):301-310. DOI: 10.1182/blood-2017-07-795047
- Durie B, Hoerin A, Sexton R, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (Pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020;10(5):53. DOI: 1038/s41408-020-0311-8
- Ito T and Handa H. Cereblon and its downstream substrates as molecular targets of immunomodulatory drugs. Intl J Hematol. 2016;104(3):293-299. DOI: 1007/s12185-016-2073-4
- Matyskiela M, Zhang W, Man H, et al. A cereblon modulator (CC-220) with improved degradation of Ikaros and Aiolos. J Med Chem. 2018;61(2):535-542. DOI: 1021/acs.jmedchem.6b01921
- Hansen J, Correa M, Nagy M, et al. Discovery of CRBN E3 Ligase Modulator CC-92480 for the Treatment of Relapsed and Refractory Multiple Myeloma. J Med Chem. 2020;63(13):6648-6676. DOI: 1021/acs.jmedchem.9b01928
- Mazahreh F, Mazahreh L, Schinke C, et al. Risk of infections associated with the use of bispecific antibodies in multiple myeloma: a pooled analysis. Blood Adv. 2023;7(13):3069-3074. DOI: 1182/bloodadvances.2022009435.
- Bhatt P, Kloock C, and Comenzo R. Relapsed/refractory multiple myeloma: A review of available therapies and clinical scenarios encountered in myeloma relapse. Curr Oncol. 2023;30(2):2322-2347. DOI: 3390/curroncol30020179
Video series
Multiple Myeloma Hub Virtual Symposium 2024: Current and future perspectives for bispecific antibodies
Your opinion matters
30 votes - 2 days left ...
Newsletter
Subscribe to get the best content related to multiple myeloma delivered to your inbox






