All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The Multiple Myeloma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Multiple Myeloma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Multiple Myeloma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Multiple Myeloma Hub cannot guarantee the accuracy of translated content. The Multiple Myeloma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Symposium | Beyond BCMA: Novel strategies using bispecific antibodies
Featured
Bookmark this article
Video series
Multiple Myeloma Hub Virtual Symposium 2024: Current and future perspectives for bispecific antibodies
During the Multiple Myeloma Hub virtual symposium held on March 11, 2024, “Current and future perspectives for bispecific antibodies in multiple myeloma: Learnings from 2023,” Amrita Krishnan, City of Hope Comprehensive Cancer Center, Duarte, US, delivered a presentation on novel strategies using bispecific antibodies beyond B-cell maturation antigen (BCMA)-directed therapies.
Krishnan discussed alternative targets for bispecific antibodies on myeloma cells, including G-protein coupled receptor family C group 5 member D (GPRC5D) and Fc receptor-homolog 5 (FcRH5), touching on a new development in dual-targeting trispecific antibodies (Figure 1). Krishnan presented clinical data from approved and investigational non-BCMA targeting agents, as well as highlighting the optimal sequencing in the myeloma treatment paradigm (Figure 2).
Figure 1. Mechanism of action of JNJ-79635322, a potential first-in-class trispecific antibody targeting BCMA, GPRC5D, and CD3
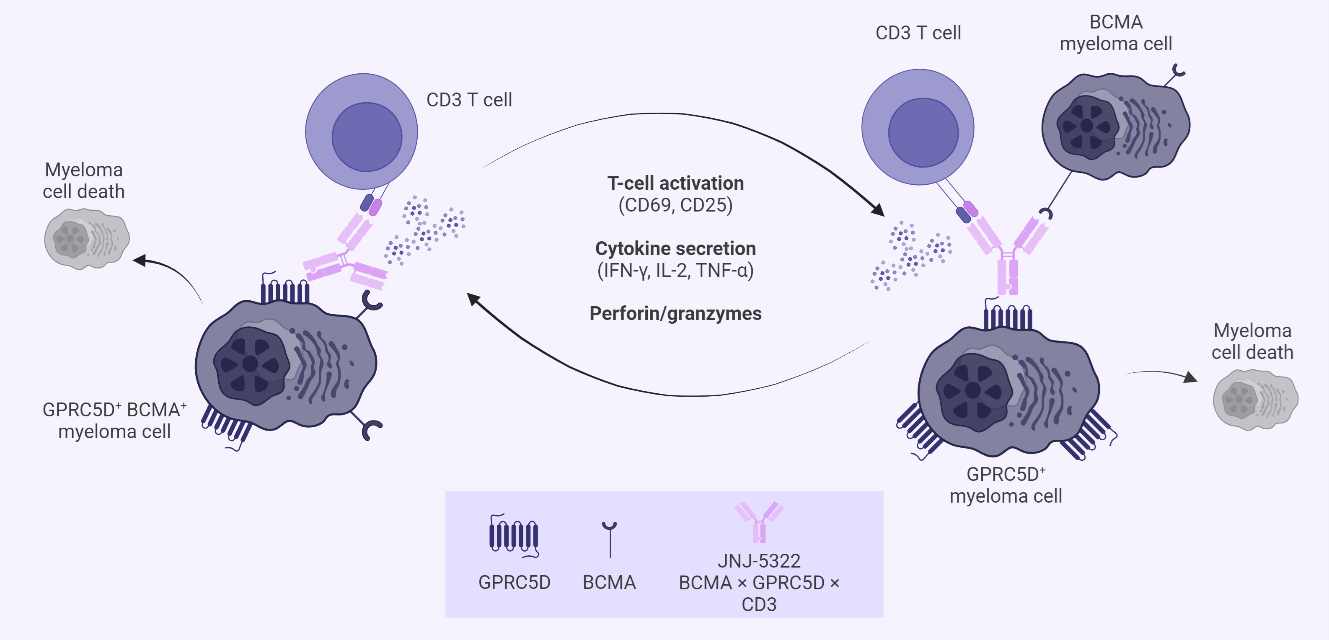
BCMA, B-cell maturation antigen; GPRC5D, G protein–coupled receptor family C group 5 member D; IFN-γ, interferon-gamma; IgG, immunoglobulin G; IL, interleukin; MM, multiple myeloma; TNF, tumor necrosis factor.
*Adapted from Pillarisetti, et al.1
Figure 2. Response rates with CAR T-cell and subsequent therapy after talquetamab in the MonumenTAL-1 trial*
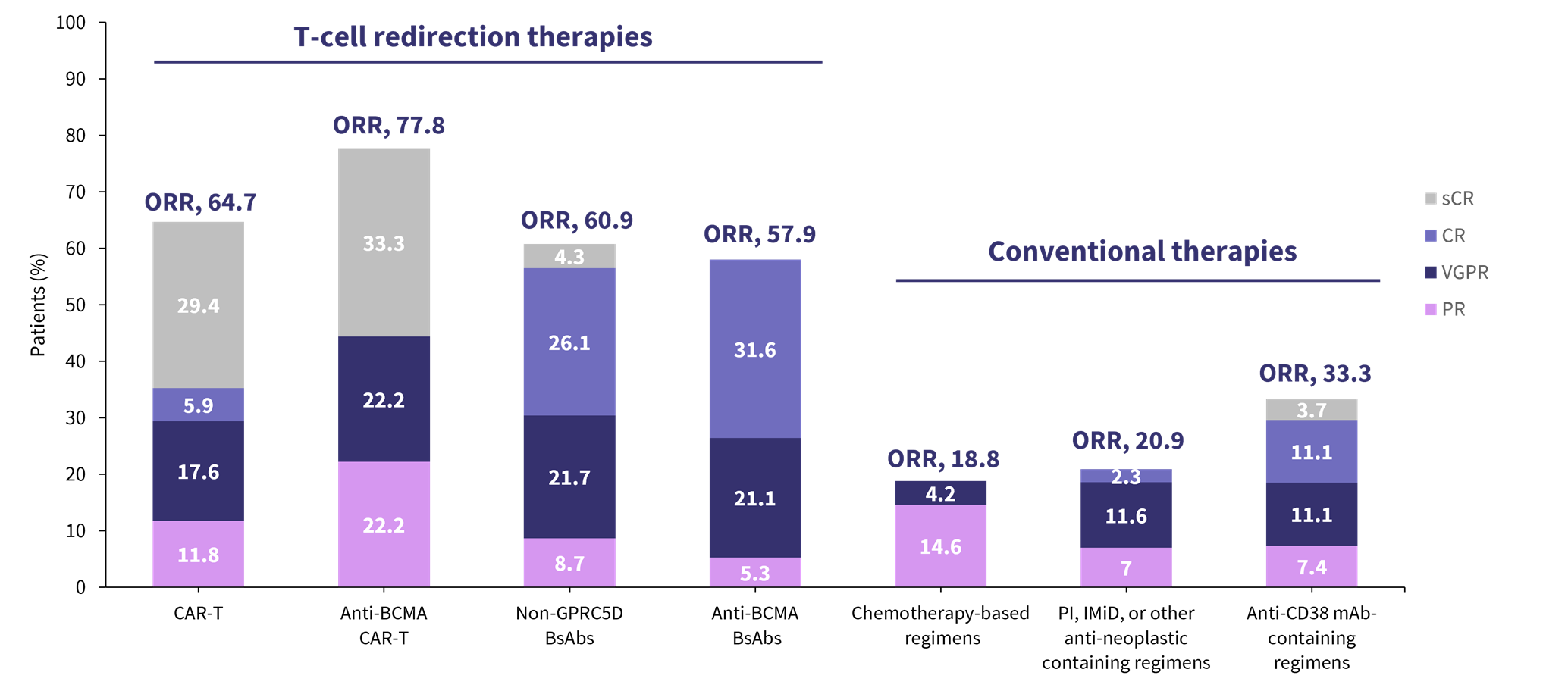
BsAb, bispecific antibody; CAR, chimeric antigen receptor; CR, complete response; GPRC5D, G protein–coupled receptor, class C group 5 member D; IMiD, immunomodulatory agent; mAb, monoclonal antibody; ORR, overall response rate; PI, proteosome inhibitor; PR, partial response; sCR, stringent complete response; VGPR, very good partial response.
*Data from Sanchez.2
This independent medical activity was funded by Janssen and Bristol Myers Squibb. All content was developed independently by the faculty. The funders were allowed no influence on the content of this activity.
- Pillarsetti K, Edavettal S, Mendonca M, et al. A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood. 2020;135(15):1232-1243. DOI: 1182/blood.2019003342.
- Sanchez L, Schinke C, Krishnan A, et al. Clinical outcomes of subsequent therapies in patients with relapsed/refractory multiple myeloma following talquetamab treatment: analyses from the phase 1/2 MonumenTAL-1 study. Blood. 2023;142 (Suppl.1):2007. DOI: 1182/blood-2023-182330
Video series
Multiple Myeloma Hub Virtual Symposium 2024: Current and future perspectives for bispecific antibodies
Your opinion matters
30 votes - 2 days left ...
Newsletter
Subscribe to get the best content related to multiple myeloma delivered to your inbox






