All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The Multiple Myeloma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Multiple Myeloma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Multiple Myeloma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Multiple Myeloma Hub cannot guarantee the accuracy of translated content. The Multiple Myeloma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
Panobinostat, gemcitabine, busulfan, and melphalan salvage therapy in RRMM
Bookmark this article
The standard-of-care for patients undergoing consolidation of frontline therapy in multiple myeloma (MM) is currently high-dose melphalan with autologous stem cell transplantation (ASCT). Progression-free survival (PFS) rates were observed to increase with the addition of busulfan (BuMel), and again with the addition of gemcitabine (GemBuMel). Individuals receiving salvage therapy often experience relapse, therefore novel combinations are continuing to be investigated for applicability in these patients.1
Here, we summarize a study published by Nieto et al.1 in American Journal of Hematology on the safety and efficacy of high-dose panobinostat combined with GemBuMel therapy following ASCT salvage in relapsed/refractory or high-risk MM.
Study design1
- Patients with high-risk or relapsed/refractory MM and adequate end-organ function were enrolled into two cohorts depending on whether they were receiving their first ASCT (ASCT-1) (n = 48) or second transplant (ASCT-2) (n = 32).
- Panobinostat was administered orally at 20 mg/day in addition to GemBuMel
- The matched control cohort for ASCT-1 (N = 299), included treatment with BuMel (n = 122) or melphalan (n = 177), and for ASCT-2, included BuMel (n = 19) or melphalan (n = 53).
- The primary endpoint was PFS
- Secondary endpoints included were overall response rate, stringent complete response, overall survival (OS), measurable residual disease (MRD), and toxicity profile.
Key findings1
- At a median follow-up of 51 months, the median PFS of the ASCT-1 and -2 cohorts were 25 months and 32 months, respectively.
- Median OS was not reached in either cohort
- Transplant-related mortality was recorded in two patients in the ASCT-2 cohort, and none in the ASCT-1 group.
- The toxicity profile was deemed acceptable, and not significantly different to the matched control groups.
- In both cohorts, MRD negativity increased following ASCT, with an improvement of 8.5% to 23% in ASCT-1, and 34% to 55% in ASCT-2
- Increased rates of MRD negativity correlated with improved outcomes
- ASCT-1 and -2 cohorts compared with matched controls:
- In both ASCT-1 and -2 cohorts, increased PFS rates were observed compared with the control groups (p = 0.025 and p = 0.04, respectively). More information and data representation can be found here
- OS rates were not significantly different in either of the matched groups
- PFS and OS rates at 1-year post-ASCT, as well as response data are presented in Figure 1.
Figure 1. 1-year PFS, OS, and response rates*
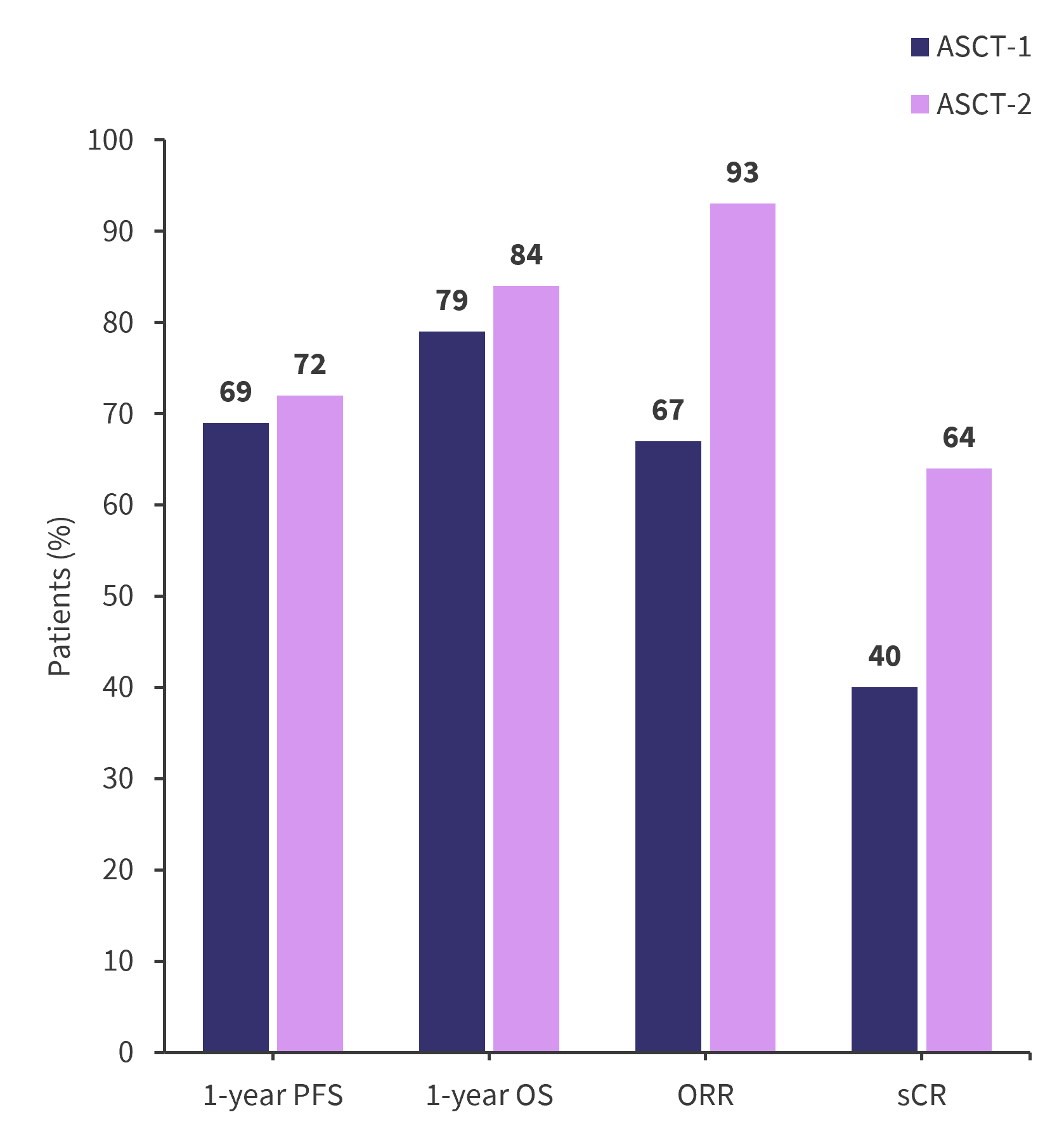
ASCT, autologous stem cell transplantation; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; sCR, stringent complete response.
*Data from Nieto, et al.1
|
Key learnings |
|---|
|
- Nieto Y, Yang Z, Valdez B, et al. Safety and efficacy of a new high-dose regimen of panobinostat, gemcitabine, busulfan, and melphalan for 1st or 2nd salvage ASCT for refractory/relapsed or high-risk myeloma: Matched-pair comparisons with concurrent control cohorts. Am J Hematol. 2024;99(2):245-253. DOI: 1002/ajh.27168
Your opinion matters
28 votes - 3 days left ...
Related articles
Newsletter
Subscribe to get the best content related to multiple myeloma delivered to your inbox






