All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The Multiple Myeloma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Multiple Myeloma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Multiple Myeloma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Multiple Myeloma Hub cannot guarantee the accuracy of translated content. The Multiple Myeloma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
European Commission approves idecabtagene vicleucel for the treatment of triple-class-exposed RRMM
Bookmark this article
On March 20, 2024, the European Commission granted approval to idecabtagene vicleucel (ide-cel) for the treatment of triple-class-exposed relapsed/refractory multiple myeloma (RRMM) with two or more prior therapies, including an immunomodulatory agent (IMiD), proteasome inhibitor (PI), and an anti-CD38 antibody with subsequent disease progression.1
In August, 2021, ide-cel was granted approval by the European Commission for the treatment of RRMM treated with three or more prior therapies, including an IMiD, PI, and anti-CD38 monoclonal antibody.2 This latest approval for ide-cel marks the first chimeric antigen receptor (CAR) T-cell therapy approved in earlier lines of therapy. This approval is based on data from the phase III KarMMa-3 (NCT03651128) clinical trial.1
The Multiple Myeloma Hub has previously reported the study design of KarMMa-3 and latest updates from ASH 2023.
KarMMa-3 – pivotal data1
- At a median follow up of 18.6 months, treatment with ide-cel demonstrated a significant increase in median progression-free survival (PFS) at 13.8 months vs 4.4 months in the comparator standard-of-care arm (hazard ratio: 0.49; 95% confidence interval [CI]: 0.38–0.63; p < 0.0001).
- The overall response rate (ORR) observed in the ide-cel arm was 71.3%, with a complete response or greater (≥CR) rate of 43.7% vs an ORR of 42.4% and ≥CR rate of 5.3% with standard-of-care treatment.
- Median overall survival was higher in the ide-cel cohort at 41.4 months vs 37.9 months.
- The safety profile observed with ide-cel was comparable to the established profile in later lines of therapy (Figure 1).
Figure 1. Incidence of CRS and neurotoxicity*
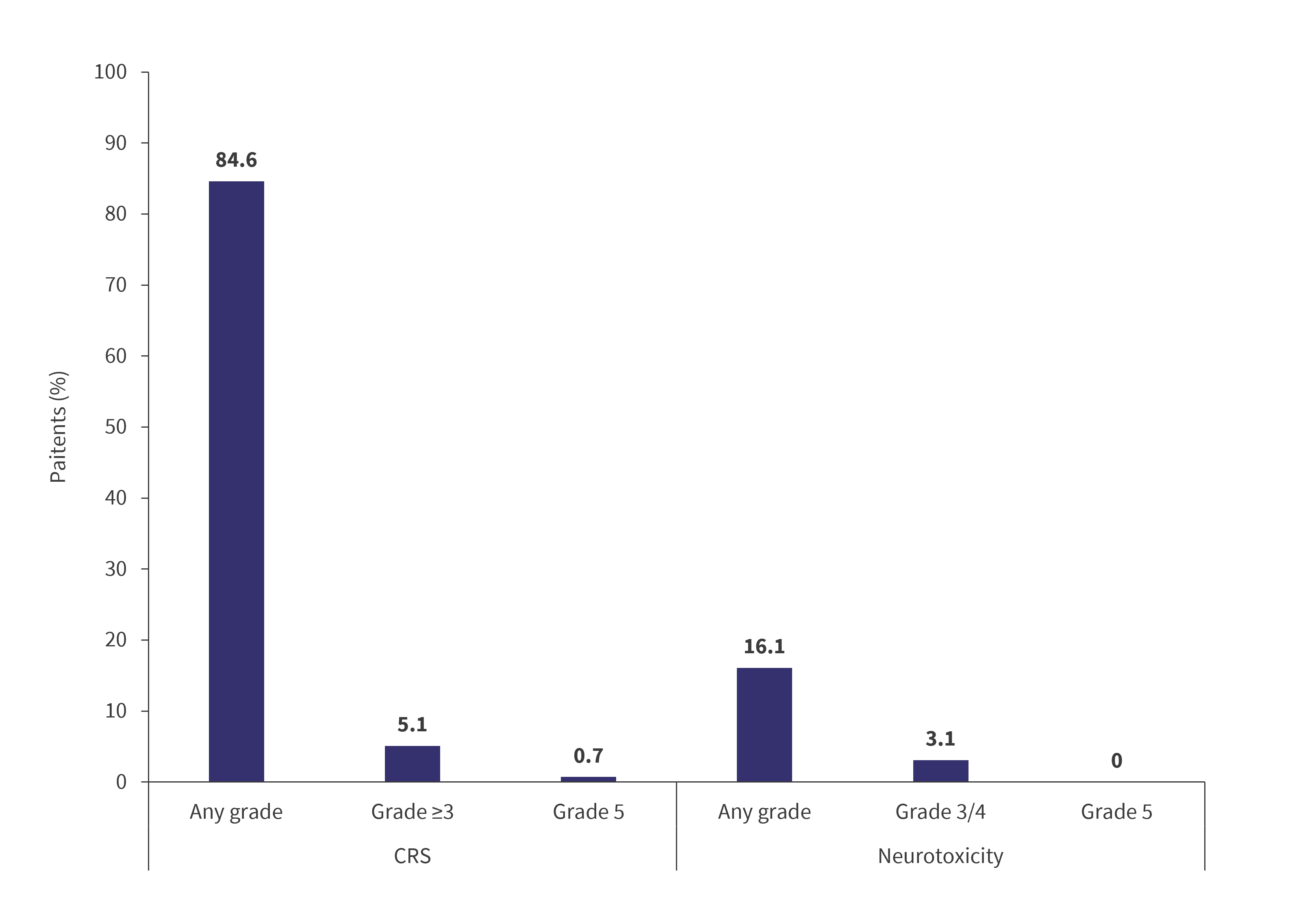
CRS, cytokine release syndrome.
*Data from Bristol Myers Squibb.1
- Bristol Myers Squibb. Bristol Myers Squibb’s Abecma (idecabtagene vicleucel) Becomes First CAR T Cell Therapy Approved in the European Union in Earlier Lines for Triple-Class Exposed Relapsed and Refractory Multiple Myeloma. https://news.bms.com/news/corporate-financial/2024/Bristol-Myers-Squibbs-Abecma-idecabtagene-vicleucel-Becomes-First-CAR-T-Cell-Therapy-Approved-in-the-European-Union-in-Earlier-Lines-for-Triple-Class-Exposed-Relapsed-and-Refractory-Multiple-Myeloma/default.aspx. Published Mar 20, 2024. Accessed Mar 21, 2024.
- Bristol Myers Squibb. Bristol Myers Squibb Receives European Commission Approval for Abecma (Idecabtagene Vicleucel), the First Anti-BCMA CAR T Cell Therapy for Relapsed and Refractory Multiple Myeloma. https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Receives-European-Commission-Approval-for-Abecma-Idecabtagene-Vicleucel-the-First-Anti-BCMA-CAR-T-Cell-Therapy-for-Relapsed-and-Refractory-Multiple-Myeloma/default.aspx. Published Aug 19, 2021. Accessed Mar 21, 2024.
Your opinion matters
28 votes - 4 days left ...
Related articles
Newsletter
Subscribe to get the best content related to multiple myeloma delivered to your inbox






