All content on this site is intended for healthcare professionals only. By acknowledging this message and accessing the information on this website you are confirming that you are a Healthcare Professional. If you are a patient or carer, please visit the International Myeloma Foundation or HealthTree for Multiple Myeloma.
The Multiple Myeloma Hub uses cookies on this website. They help us give you the best online experience. By continuing to use our website without changing your cookie settings, you agree to our use of cookies in accordance with our updated Cookie Policy
Introducing

Now you can personalise
your Multiple Myeloma Hub experience!
Bookmark content to read later
Select your specific areas of interest
View content recommended for you
Find out moreThe Multiple Myeloma Hub website uses a third-party service provided by Google that dynamically translates web content. Translations are machine generated, so may not be an exact or complete translation, and the Multiple Myeloma Hub cannot guarantee the accuracy of translated content. The Multiple Myeloma Hub and its employees will not be liable for any direct, indirect, or consequential damages (even if foreseeable) resulting from use of the Google Translate feature. For further support with Google Translate, visit Google Translate Help.
CARTITUDE-2 cohorts A and B: Updated efficacy and safety results
Bookmark this article
The phase II CARTITUDE-2 trial (NCT04133636) evaluated safety and efficacy of the B-cell maturation antigen-directed chimeric antigen receptor T-cell agent ciltacabtagene autoleucel for treatment of relapsed/refractory multiple myeloma.
During 2024 Transplantation & Cellular Therapy Meetings of the ASTCT and CIMBTR (TANDEM), Hillengass.1 presented updated results from the CARTITUDE-2 trial. We summarize the key results below.
Study design1
- Cohort A: patients with 1–3 prior lines of therapy
- Cohort B: patients who relapsed at ≤12 months after transplant/at antimyeloma treatment initiation if without transplant
- All patients received a target dose of 0.75 × 106 cells/kg.
- The primary endpoint was negative measurable residual disease at a sensitivity of 10−5.
Key findings1
- Cohort A = 20 patients
- Cohort B = 19 patients
- Median follow-up 29 months
- Most patients across both cohorts achieved negative measurable residual disease (Figure 1).
Figure 1. Rates of overall and sustained negative MRD in cohorts A and B from CARTITUDE-2 l*
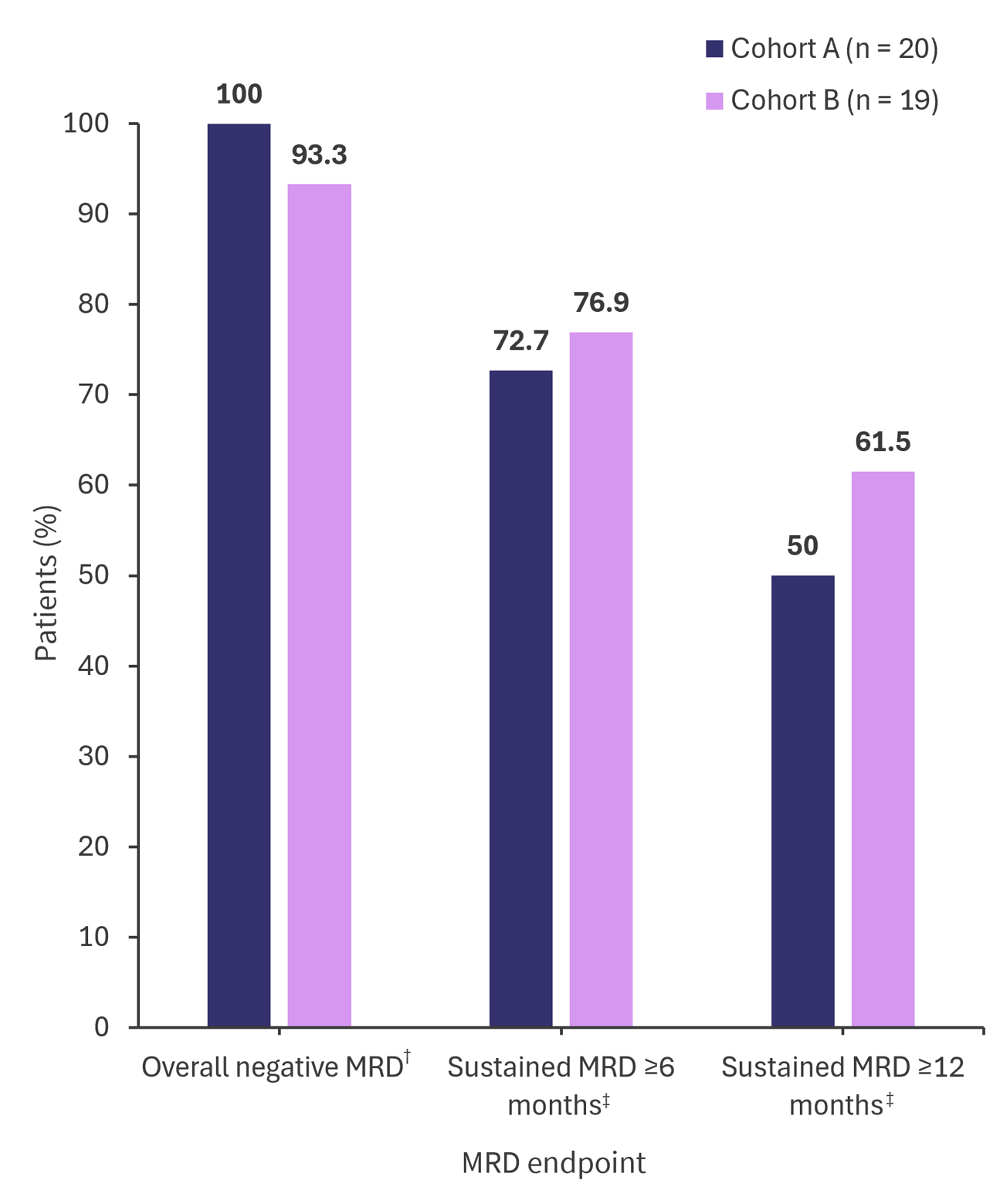
MRD, measurable residual disease.
*Adapted from Hillengass.1
†Cohort A (n = 17), cohort B (n = 15).
‡Cohort A (n = 11), cohort B (n = 13).
§Cohort A (n = 14), cohort B (n = 13).
- Duration of response, overall survival and progression free survival rates at 24 months were comparable between the two patient cohorts (Table 1).
Table 1. ORR and OS, PFS and DOR rate at 24 months*
|
Cohort A |
Cohort B |
|
|---|---|---|
|
ORR, % |
95 |
100 |
|
OS at 24 months, % |
75.1 |
84.2 |
|
PFS at 24 months, % |
75 |
73.3 |
|
DOR at 24 months, % |
73.3 |
70.5 |
|
DOR, duration of response; ORR, overall response rate; OS, overall survival; PFS, progression free survival. |
||
- Most Grade 3/4 hematologic treatment related adverse events were experienced by more patients in cohort A vs cohort B (Table 2).
Table 2. Cytokine release syndrome, ICANS, and Grade 3/4 hematologic TRAE*
|
%, unless otherwise stated |
Cohort A |
Cohort B |
|---|---|---|
|
Any grade CRS |
95 |
84.2 |
|
Any grade ICANS |
15 |
5.3 |
|
Hematologic TRAE |
||
|
Neutropenia |
95 |
89.5 |
|
Lymphopenia |
80 |
47.4 |
|
Thrombocytopenia |
40 |
26.3 |
|
Anemia |
45 |
47.4 |
|
Leukopenia |
60 |
31.6 |
|
CRS, cytokine release syndrome; ICANS, immune cell associated neurotoxicity syndrome, TRAE, treatment-related adverse event. |
||
|
Key learnings |
|---|
|
- Hillengass J. The phase 2 Cartitude-2 trial: Updated efficacy and safety of ciltacabtagene autoleucel in patients with multiple myeloma and 1–3 prior lines of therapy (cohort A) and with early relapse after first line treatment (cohort B). Oral abstract #42. 2024 Transplantation and Cellular Therapy Meetings of the ASTCT and CIMBTR; Feb 23, 2024; San Antonio, US.
Your opinion matters
28 votes - 4 days left ...
Related articles
Newsletter
Subscribe to get the best content related to multiple myeloma delivered to your inbox






